A Leap Forward In The Fight Against Corrosion
Understanding corrosion.
Corrosion is defined as the degradation or destruction of metals and alloys in an environment by the process of electrochemical or chemical process. It affects all aspects of industry; automotive, construction, transportation, production, marine and manufacturing to name a few. A recent study by NACE International found corrosion costs $2.5 trillion globally, contributing to 3.4% of gross domestic product.
The term corrosion can be applied to the deterioration of plastics, concrete and wood, but is mainly used to define the chemical break down of steels (containing a high iron content).
There are five main types of corrosion:
- Uniform corrosion: whereby the surface layer of metal/alloy will rust due to environmental factors. This type of corrosion is most common amongst ferrous metals and alloys which are uncoated.
- Crevice corrosion: this form of corrosion is usually associated with a stagnant solution within the formed crevice of the metal. It is initiated through ionic changes in the crevice; changes in acid potential, a build up of ion species and a depletion of oxygen.
- Galvanic corrosion: this type of corrosion is driven by corrosion potentials of dissimilar metals when in an electrolytic solution (water). The lowest corrosion potential metal becomes the anode and the highest becomes the cathode.
- Electrolytic corrosion: similar to galvanic corrosion, the only difference being that the corrosion does not have to take place in an electrolytic solution. There must be an external source of electromotive (EMF), this could be from a battery or electric generator etc.
- Filiform corrosion: this is noticeable by a ‘bubbling’ effect on metals which are protected by lacquers, paints or coatings. The protective coating becomes susceptible to breakdown and the corrosion builds up underneath the protective layer.
The corrosion process is known as a reduction-oxidation (redox) reaction; the oxidation reaction takes place at the anode and the cathode is protected during the reduction reaction. This reaction requires four prerequisites:
- An anode – negatively charged electrode which discharges negative ions (anions), forming into positive ions
- A cathode – positively charge electrode which discharges positive ions (cations), forming into negative ions
- An electron pathway – the flow of electrons from one electrode to another
- An electrolyte – a substance which produces an electrically conducting solution when dissolved in a polar solution, i.e. water.

When considering the above process, it is helpful to visualise the reaction as part of an electrochemical cell:
There are four factors which control the rate in which corrosion occurs, these are as follows:
- pH of the environment; an increase in corrosion occurs when the pH falls below 4.
- Oxygen content; an increase in oxygen increases the rate of corrosion.
- Flow rate; increased flow of water and oxygen to the surface removes protective surface films and increases corrosion.
- Water type; natural waters (soft water) contains a higher concentration of chloride ions and sulphate ions which can interact with the surfaces of metals more readily.
There are a few ways which corrosion can be prevented, such as; coating/anodizing the metal surface, using corrosion inhibitors in cavities, removing the access of oxygen and controlling the electrochemical potential of metals in contact with each other. However, these methods may be time consuming, and expensive for mass production processes.
How can you protect your vehicle from corrosion?
Protecting your vehicle from corrosion can be extremely difficult, which is why Forgeway have developed a new anti-corrosive spray adhesive (Formoa 760-AC) to protect any metal from the electrochemical process of corrosion. This product has been developed to protect under vehicle bodies, cavity protection and areas which are at risk of water entrapment. It works by omitting a chemical around the surface of the metal where the adhesive has been applied and inhibits the redox reaction on the metals surface, thus prolonging and preventing the breakdown of the protective layer on the metal surface.
See the results.
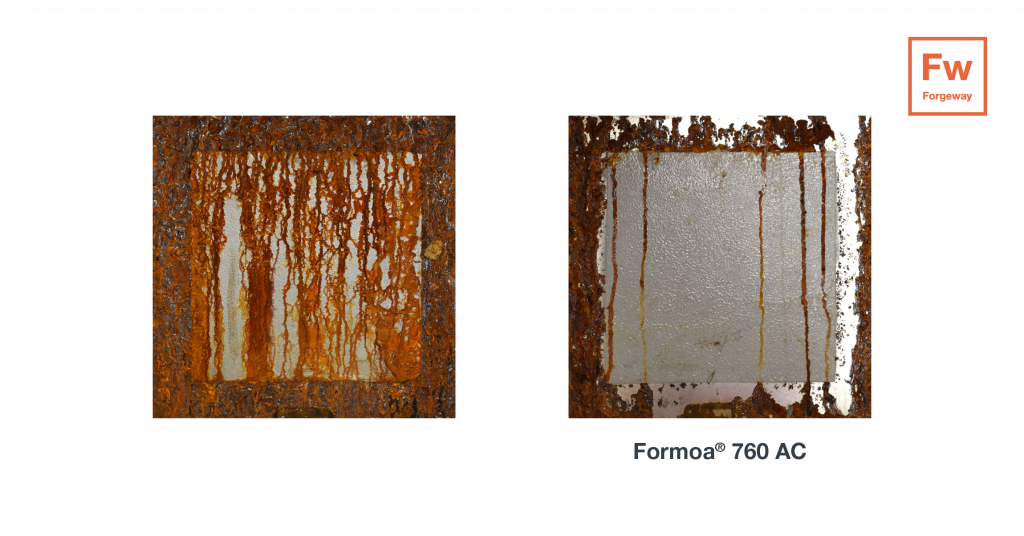
The testing was conducted on 300 X 300 X 3mm (H X W X D) mild steel plates; mild steel has a high iron content, meaning its corrosion potential is high and will therefore corrode at a faster rate. Formoa 760-AC was sprayed onto the mild steel to coat the entire back of the plate. Then sprayed in a 200 X 200mm square on the front of the plate, this was so bare steel was exposed during testing. This process was repeated with standard Formoa 760 (containing no anti-corrosive inhibitor). The plates were extensively tested in a salt spray chamber for 500 hours, the parameters for testing are listed below:
- Test Standard: ASTM B117-11.
- Temperature of chamber: 35°C
- Temperature of air saturator: 49°C
- Salt concentration: 5% NaCl +/-1%
- Samples were positioned 15 degrees from vertical

The images below show the dramatic improvement of the bare mild steel after 500 hours in salt spray and show the beneficial effect of using Formoa 760-AC as a protective coating on metals.

When taking into consideration the total cost corrosion has on industry, there is no time to waste when ensuring that your vehicles are protected from corrosion and increasing their time on the road. You can depend on Formoa 760-AC and be rest assured that your vehicles will be rust free.
Interested in learning more? Head over to our YouTube Channel for a range of videos on all things adhesives and bonding.
Topics:


